Science >Physics >Heat Transfer > Conduction
- Heat conduction is the transfer of energy between neighboring molecules in a substance due to a temperature gradient. In metals also the free electrons transfer energy. In solids which do not transmit radiation, heat conduction is the only process for energy transfer.
- Important even in situations in which there is an intervening medium; a familiar example is the heat transfer from a glowing piece of metal or from a fire. Muddy points How do we quantify the contribution of each mode of heat transfer in a given situation? (MP HT.1) 2.0 Conduction Heat Transfer We will start by examining conduction heat transfer.
- If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with the slower ones with a net transfer of energy to the slower ones.
- Since one iron rod is twice the diameter of the other, it has four times the cross-sectional area, and thus should conduct heat four times as fast. It also has, however, four times the mass of the other rod, and so requires four times as much heat to raise its temperature by the same amount.
Heat always gets transferred from the body and higher temperature to a body at lower temperature heat transfer can take place in three ways a) Conduction b) Convection and c) Radiation. In this article, we shall study the heat transfer by the conduction.
Conduction:
A noninsulated uniform rod positioned between two walls of constant but different temperature. The finite-difference representation employs four interior nodes. Where T =temperature ( C), x =distance along the rod (m), h′ =a heat transfer coefficient between the rod and the surrounding air (m−2), and T a = the air temperature ( C).
If one end of a metal rod is heated, the other end also gets heated up. This is due to conduction. When one end of a metal rod is heated, the kinetic energy of the molecules at that end increases. The molecules start vibrating with a higher amplitude. These molecules start vibrating with a higher amplitude. These molecules during vibration collide with the neighbouring molecules and transfer part of their energy to the neighbouring molecules. Thus the kinetic energy of the neighbouring molecules increases hence their amplitude of vibration increases and during the collision the energy transfers to the next molecule. Thus heat transfer takes place by conduction.
The modeof heat transfer between two parts of a body or between two bodies in contactwhich are at different temperatures without actual migration of particles ofthe body is called conduction.
Dependingupon easiness of heat transfer by conduction the substance are classifieds intotypes a) Good Conductors and b) Bad conductors
Good Conductors:
Thesubstances which allow the heat to pass through them very easily are calledgood conductors. Examples. Aluminum, copper, Silver, Steel, Bronze, Brass, allmetals
Bad Conductors:
Thesubstances which do not allow the heat to pass through them are called badconductors. Bad conductors of heat are also called as insulators. Examples:wood, rubber, Plastic, paper, glass, air, ebonite , bakelite.
Use of conduction:
- Metals are used for making utensils because the metals are good conductors of heat they allow heat to pass through them easily.
- Cooking vessels have plastic handles because plastic a bad conductor of heat it does not allow the heat to pass through from hot vessel to hands and thus danger of burning can be avoided.
- Tea-cups, Teapots, coffee jugs are made of porcelain.
- Mountaineers use sleeping bags in polar regions.
- People wear woolen cloth in winter.
- Nowadays cooking vessels are made with copper bottoms.
- In winter, the metal lock feels colder than the wooden door on touch.
Characteristics of conduction:
- In this type of heat transfer, there is no actual migration of the medium particles from one point to another.
- For conduction, there must be a material contact between the two bodies.
Heat Transfer In An Iron Rod
Concept of Steady-State and Temperature Gradient:
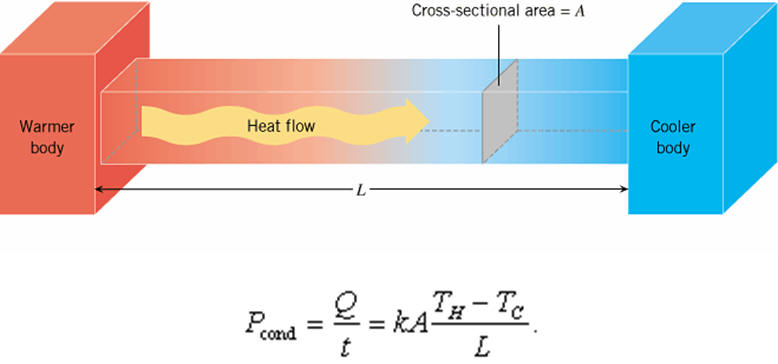
Heatconduction may be described quantitatively as the time rate of heat flowin a material for a given temperature difference.
Consider ametallic bar AB of length L and uniform cross-sectional area A withits two ends maintained at different temperatures. The temperaturedifference between the ends can be obtained by keeping the ends in thermalcontact with large reservoirs having temperature differences. Some holesare drilled on this rod to insert thermometers (say T1, T2,T3, and T4) in the rod. For better thermal contactbetween the rod and thermometers mercury is poured into the holes. The sides ofthe bar are fully insulated so that no heat is exchanged between thesides and the surroundings.
Let θ1, θ2, θ3, and θ4 be the temperatures recorded by the thermometers T1, T2, T3, and T4 respectively. Initially, the temperature rises and after some time every thermometer shows its own constant reading such that (θ1 > θ2 > θ3> θ4). This state is called the steady-state.
Due to the insulation of the rod, no heat is lost due to surroundings. At a steady-state, at every cross-section of the rod, the quantity of heat entering the section in one second is equal to the quantity of heat leaving the section due to conduction.
Let usconsider two sections separated by distance Δx and let Δθ be thetemperature difference between these two sections. then the quantity Δθ/ Δx is called the temperature gradient.
The temperature gradient is defined as the rate of change of temperature with the distance when the material is in steady-state.
Thermal Conductivity:
It is found experimentally that in this steady state, the rate of flow of heat (or heat current)H is proportional to the temperature difference (θ2 – θ1) and the area of cross-section A and is inversely proportional to the length L
Where K = Constant called the thermal conductivity or the coefficient of thermal conduction the material. The greater the value of K for a material, the more rapidly will it conduct heat.
The SI unitof K is J S–1 m–1 K–1 (jouleper second per metre per kelvin) or W m –1 K–1 (wattper metre per kelvin).
The value ofthermal conductivity varies slightly with temperature but can be considered tobe constant over a normal temperature range. Good thermal conductors havevery high values of thermal conductivity while thermal insulators havenegligible values of thermal conductivity.
Houses made of concrete roofs get very hot during summer days because the thermal conductivity of concrete (though much smaller than that of metal) is still not small enough. Therefore, a layer of earth or foam insulation is put on the ceiling so that heat transfer is prohibited and the room remains cooler.
Searle’s Experiment:
Apparatus:
Apparatusconsists of the thermally insulated box housing a metallic bar of auniform cross-sectional area with its one end kept in contact withsteam in a steam chamber. Two holes are drilled to insert thermometers T1 and T2,in the rod separated by distance x. For better thermal contact between the rodand thermometers mercury is poured into the holes. Cooling water is circulatedaround the rod whose initial and final temperatures are measured by thethermometers T3 and T4.
Working and Calculations:
At steady state, the heat lost by rod = heat gained by the water
Heat Transfer In Metal Rodent
Where, mW = Mass of water, SW =Specific heat of water, t = time for which heat is flowing
Measuring all values on R.H.S. of the formula value of K canbe found.
Values of thermal conductivity in J S–1 m–1 K–1 fordifferent materials are given below